Background: Acute myeloid leukemia (AML) is a devastating disease difficult to treat. Although > 60% of patients (pts) with AML treated with standard therapy achieve complete remission, at least 70% relapse. Overexpression of lysine-specific demethylase 1 (LSD1) mRNA and excess LSD1 protein have been observed in several cancer types, including progenitor populations of leukemic cells in AML. In preclinical studies, LSD1 inhibition impaired tumor growth and induced apoptosis of leukemic cells (Harris et al., 2012). In combination with ATRA, LSD1 inhibition enhanced survival in mouse models of AML (Shenck et al., 2014). In this first-in-human trial, we examine the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of bomedemstat with or without ATRA in pts with high-risk AML and myelodysplastic syndrome (MDS).
Methods: In this multicenter, open-label phase 1/2a trial, eligible pts were aged ≥18 years old, with high-risk AML or MDS, ECOG PS ≤2 (AML), and International Prognostic Score (IPSS) of at least intermediate-2 or Revised International Prognostic Score (IPSS-R) of at least intermediate (MDS). Pts could not have received immunotherapy within <8 weeks, chemotherapy within <3 weeks (except hydroxyurea), and radiation to >30% of marrow within <2 weeks of treatment. In the dose-finding phase, pts received bomedemstat at a starting dose of 0.75, 1.5, 3.0, or 6.0 mg/kg once-daily in 14-day cycles (7 days on, 7 days off), either alone or in combination with 45 mg/m 2ATRA for up to 4 cycles. Treatment was allowed to continue upon evidence of clinical benefit. In the duration-finding phase, pts received 6.0 mg/kg bomedemstat with ATRA in 21-day (14 days on, 7 days off) or 28-day (21 days on, 7 days off) cycles. Treatment continued until progression, unacceptable toxicity, or withdrawal. Primary endpoint was safety and tolerability. Secondary endpoints included PK parameters and PD effect on hematopoiesis. Clinical responses were documented per revised/modified Cheson criteria. Data cut-off date was December 13, 2018.
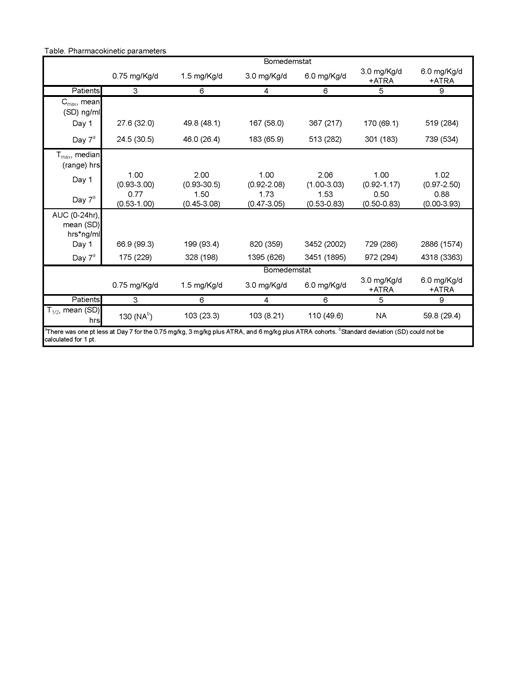
Results: A total of 45 pts enrolled with a median age 73 years (range, 42 to 88); 19 (42%) were female. A total of 39 pts had AML with 27 (69%) having an ECOG PS score ≤1. Six pts had MDS with 2 (33%) pts having an IPSS of Very High Risk and IPSS-R of High Risk. A total of 33 pts were enrolled in the dose-finding phase (0.75 mg/kg/d bomedemstat [n=3], 1.5 mg/kg/d [n=6], 3.0 mg/kg/d [n=4], 6.0 mg/kg [n=6], 3.0 mg/kg + ATRA [n=5], 6.0 mg/kg + ATRA [n=9]). Twelve pts enrolled in the duration finding phase, 4 received bomedemstat plus ATRA for 14/28 days, and 8 received bomedemstat plus ATRA for 21/28 days. A total of 21 (47%) pts discontinued, 8 (18%) due to death, 5 (11%) due to withdrawal and 4 (9%) due to physician decision. Any grade adverse events (AE) related to bomedemstat occurred in 42 (93%) pts. The most common were diarrhea (42%), nausea (42%), and thrombocytopenia (38%). Grade ≥3 bomedemstat-related AEs occurred in 30 (66.7%) pts. One pt receiving 6.0 m/kg plus ATRA had a grade 5 AE related to bomedemstat due to respiratory failure. No patient experienced dose-limiting toxicity and no maximum tolerated dose was determined. The table summarizes PK parameters in whole plasma for the dose-finding phase. Based on plasma exposures, the Data Safety Monitoring Committee designated 6.0 mg/kg bomedemstat as the PD dose. Mean percent change (standard deviation) in blast counts was 368% (400) for pts receiving 0.75 mg/kg bomedemstat, 2478% (3279) for 1.5 mg/kg, 10% (25) for 3 mg/kg, 2841% (6047) for 6 mg/kg/d. For pts with AML, overall median EFS was 0.97 months (mo) (95% CI, 0.93-1.46), and median OS was 18.8 mo (95% CI, 3.86 to NR). The 6-mo rate for EFS and OS were 9% (95% CI, 0-46%) and 64% (95% CI, 25%-86%), respectively. The overall response rate was 28.2% (95% CI, 15.0-44.9, [1 PR, 10 SD]). For pts with MDS, median EFS was 0.93 mo (95% CI, 0.57 to NR) and median OS was NR (95% CI, 0.57 to NR). One pt with MDS had SD as best response (95% CI, 0.4-64.1).
Conclusions: Bomedemstat up to 6.0 mg/kg alone or in combination with ATRA was well-tolerated in pts with high-risk AML or MDS. Change in blast count seen in treated pts could be due to differentiation to monocytes induced by bomedemstat rather than blast count reduction per se. Objective responses were seen in a subset of pts with AML (28%). OS was numerically higher to historical data with standard of care. Bomedemstat is currently under investigation in myeloproliferative neoplasms.
Disclosures
Ross:Takeda: Membership on an entity's Board of Directors or advisory committees; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Consultancy; Menarini: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Research Funding. Stevenson:Imago Biosciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding. Shortt:Otsuka: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Speakers Bureau; Mundipharma: Consultancy, Speakers Bureau; Astellas: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Amgen: Research Funding; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding. Cooney:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding. Lewis:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding. Rienhoff Jr.:Imago Biosciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Ended employment in the past 24 months.